In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of current and former FDA reviewers, scientists, engineers and regulatory and quality experts, and adds additional expertise with FDA submissions. The author of this post is a member of this team, which has done significant work with novel and/or high-risk devices focusing on pre-submissions, 510(k)s, IDEs, PMAs, De Novos, Breakthrough Designation Requests and Safer Technology Program Requests.
FDA has just released a draft guidance for bone anchors. This new draft guidance document, dated January 3, 2017, is a reissuance of the previous version from April, 1996: "Specifically, this guidance reflects the most current thinking on relevant bench testing methods for bone anchor devices including nitinol and absorbable polymeric bone anchors."
Bone anchors! These cool medical devices help attach soft tissue to bone. Partial or full detachment from the bone of ligaments, tendons and other tissues, are common injuries. Think achilles tendons, rotator cuff tears and other shoulder injuries, ACL tears and other knee injuries, and countless other ailments. While these tissues can re-attach without intervention, in some cases, especially with complete detachment, surgery may be necessary to help the process along. Bone anchors assist with these surgeries by, literally, anchoring a suture to the bone from the tissue. The injury will then heal as the as the connection between bone and tissue is reestablished.
Have a look:
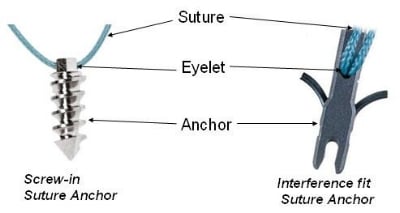
Photo from dolcera.com.
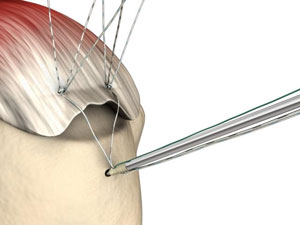
Photo from smith-nephew.com.
This video provides a pretty cool illustration of how it works:
And this video provides a different angle and shows a different type of anchor:
Some of these anchors, like some of the sutures they help affix, are dissolvable. It's always impressive to encounter a device that can do its job and then quietly disappear. The new draft guidance focuses on this area, and the Office of the Federal Register explains, "The guidance provides recommendations for the information and testing that should be included in premarket submissions for bone anchor (suture anchor) devices used in the appendicular skeleton for attachment of soft tissue to bone. This draft guidance is not final nor is it in effect at this time." Comments are being accepted through March 6, 2017, and the link above to the Federal Register explains how they can be submitted.
Further reading:
Corresponding MAI product code
Code of Federal Regulations for smooth or threaded metallic bone fixation fastener


