In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of current and former FDA reviewers, scientists, engineers and regulatory and quality experts, and adds additional expertise with FDA submissions. The author of this post is a member of this team, which has done significant work with novel and/or high-risk devices focusing on pre-submissions, 510(k)s, IDEs, PMAs, De Novos, Breakthrough Designation Requests and Safer Technology Program Requests.
Imagine you have a condition where your body doesn't produce enough of the chemical that keeps your blood vessels open and clear, instead allowing them to thicken. This of course impacts the amount and flow of oxygen you're getting and thus affects your heart and lungs.
Imagine that there is, fortunately, a treatment for this. But treatment requires regular visits to medical professionals for IV drips or shots, to keep your body functioning and battling this condition. You go because these visits are helping keep you alive. But what if you could just have the whole system implanted and skip most of those visits, constant monitoring, frequent skin punctures, and some of the possible side effects? Well, now you can.
Pulmonary arterial hypertension (PAH) is a progressive disease where they body doesn't make enough prostacyclin, which allows the pulmonary system to function properly. Remodulin (treprostinil) is a drug that can treat this condition, but which needs to be regularly administered. A new system recently approved by FDA allows the whole thing to be implanted and doses administered automatically via an IV catheter, thus improving quality of life: the Implantable System for Remodulin is a fully implantable system designed to deliver Remodulin® (treprostinil) injection intravenously for the treatment of patients with pulmonary arterial hypertension (PAH).
Remodulin is stored in the pump reservoir and, per a programmed prescription, moves through the pump tubing, the catheter port, and the catheter to the intravascular delivery site. The programmer is a handheld device that is used to review and program pump parameters using telemetry, a radio frequency (RF) communication. The physician or healthcare provider usually calibrates this at a first visit. Subsequent visits are of course required, but the level of maintenance versus routine IV drips or injections is considerably less.
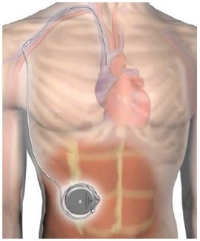
Image from medgadget.com.
The American College of Cardiology sums it up well: "The most notable benefit of the implantable system is that it significantly reduces the rate of BSIs [blood stream infections] and site infections versus that associated with external IV systems. It also eliminates chronic treprostinil-induced infusion site pain associated with SC administration. By reducing the rate of catheter-related complications and pump user errors that cause drug interruptions or overdose, it improves the consistency of therapy. Finally, it improves patient's quality of life by eliminating the daily burden of system management, and reducing restrictions and fear during normal activities of daily life. The majority (93%) of patients rate the implantable system as "very good" or "excellent"."
The Implantable System for Remodulin, which is marketed by Medtronic, is made up of 3 parts:
- The pump (Medtronic SynchroMed II 8637P Programmable Pump)
- The catheter (Medtronic 8201 Implantable Intravascular Catheter)
- The programmer (Medtronic N’Vision 8840 Clinician Programmer with 8870 Application Card)
The pump is implanted by the surgeon in the subcutaneous pocket using an incision in the lower abdomen. The intravascular catheter is connected to the pump and inserted through a vein at the superior caval-atrial junction which is the junction between the superior vena cava and the right atrium of heart. The pump reservoir remains permanently implanted and the health care provider uses a needle and syringe refill kit to refill the pump with Remodulin by a percutaneous procedure.
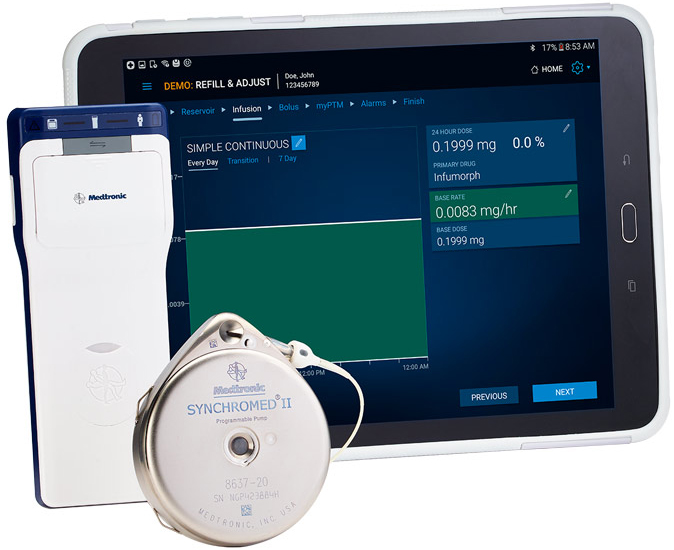
Image from medgadget.com.
This medical device does come with some parameters. Restrictions for The Implantable System for Remodulin should not be used in the following patients, including NYHA Class IV heart failure patients; patients who cannot tolerate a sudden cessation of Remodulin therapy; patients with a known or suspected infection, bacteremia, or sepsis requiring antibiotics; patients with vasculature that is inadequate for an 8 French introducer or catheter advancement without stylet guidance; patients implanted with leads or catheters (active or abandoned) in the superior vena cava that cannot be removed prior to or at system implant; patients whose body size is not sufficient to accept pump bulk and weight; patients with skin or soft tissue that would heal poorly, increase susceptibility to infections, or is unacceptable for implant of this system; patients for which a health care provider cannot implant the pump 2.5 cm or less from the surface of the skin.
It's interesting and exciting to see how medical devices can make for new versions of existing successful therapies. This implantable system is a great example of how quality of life can be improved via medical device technology without having to reinvent the proverbial wheel.


